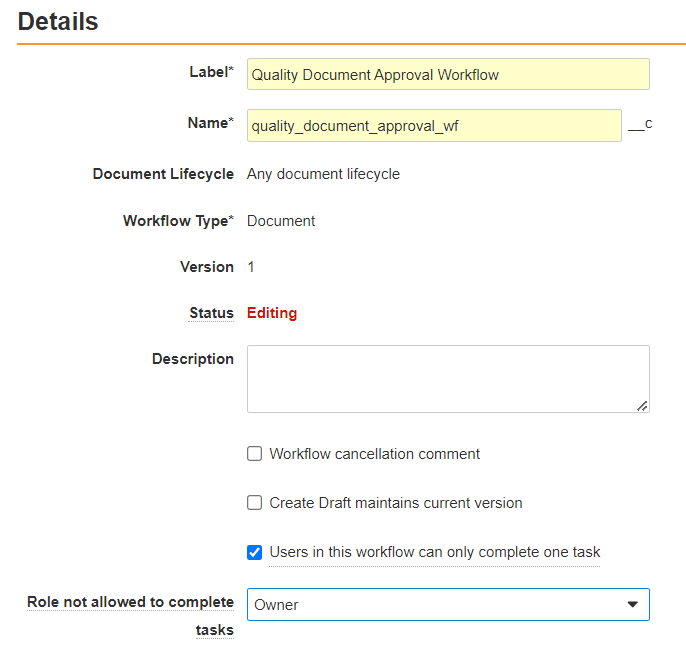
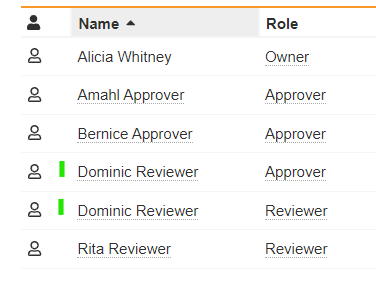
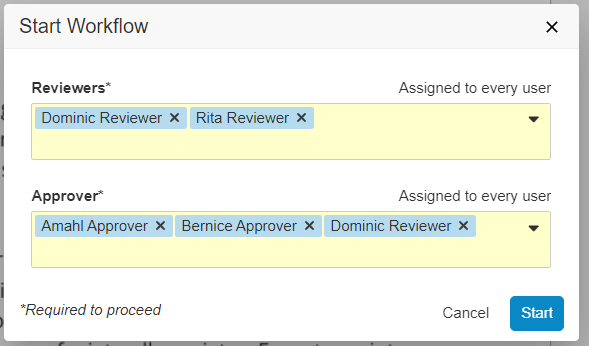
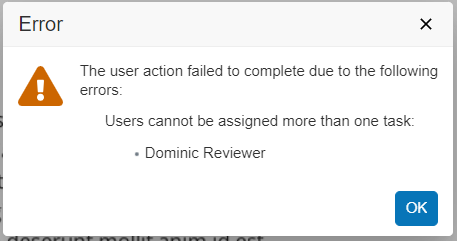
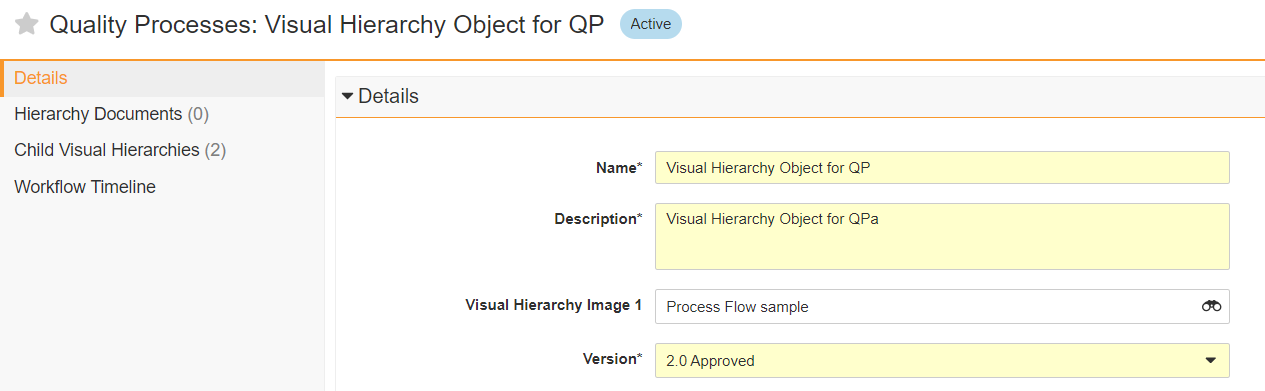
Solutions for the CARA Platform at Generis Generate 2024
fme is excited to be presenting our CARA Platform success stories and solutions at Generate 2024 in Lisbon, Portugal this April. As a certified Generis partner, we’ll be contributing to the discussions and sessions focused on maximizing the value of The CARA Platform and The CARA Life Sciences Platform, specifically built for Life Sciences applications.
Here are a few of the topics we’ll be sharing. Contact us below to schedule a custom presentation on how fme can serve your CARA Platform needs.
Successful Migrations to The CARA Platform
The fme team are experts at migrating data, documents and content into, out of, and within the leading content management platforms in the most complex regulated industries. We bring all our experience and know-how to The CARA Platform and have earned our Certified Partner badge year after year.
Whether you are moving to CARA for the first time, consolidating repositories, or upgrading to the newest release, fme understands the possibilities and pitfalls of the platform, and can ensure you have a smooth transition that delivers the maximum ROI while minimizing downtime. Our business consulting and technology services teams work together to provide end-to-end CARA implementation support to ensure your configuration and integration is effectively aligned with your business processes and goals.
Custom-built Tools and Solutions
fme has built a portfolio of proprietary tools and services to streamline and accelerate migration initiatives that we apply to CARA Platform initiatives. Each of these solutions has been tested, refined, and proven through years of client projects.
migration-center™: Save you 60% in costs and 80% in project duration with migration-center, our proprietary migration tool that is CARA ready, allowing you to minimize risks while migrating from any source system into CARA with ease. Learn more >>
MetadataAssist℠: Leverage the power of guided AI and NLP to identify and rescue disconnected content. MetadataAssist supplements your team’s expertise to accelerate the analysis, identification, categorization, and updating of metadata on millions of documents in a fraction of the time. We ensure all your information is fully organized and accessible. Learn more >>
HealthCheckAssist℠ for Generis: Don’t guess at how to improve – HealthCheckAssist tells you where you are and where you should go next based on your own priorities and business goals. Our experts analyze and evaluate your current system and provide a prioritized roadmap for best next steps to enhance efficiency and effectiveness. Learn more >>
PlatformAssist℠ for Generis: Reduce your platform management costs. PlatformAssist provides in-depth vendor release management, application change management, application and admin support and more for carefree, cost-effective management of your CARA platform. Learn more >>
compliance-center℠: Streamline, simplify, and accelerate your validation workflows. Built on the CARA platform and based on the CSA guidelines plus our 25 years of working with global pharmaceutical firms, compliance-center’s intuitive interface and clear instructions guides you through your validation process, providing detailed direction on when you can take advantage of CSA, when traditional CSV is required, and when a hybrid approach is possible. Learn more >>
Let’s Connect at Generate 2024!
If you are attending Generate, contact us so we can connect at the booth and join us for our VP of Business Consulting David Gwyn’s presentation on Insights and Best Practices for Maximizing the Value of The CARA Platform. We look forward to seeing you there!
Not attending? No Problem!
If you aren’t able to attend Generis Generate this year, we still have you covered! Connect with us below and we’ll send you the conference slides, and schedule a customized introduction to our CARA Platform solutions.
 fme AG
fme AG fme SRL
fme SRL