Maximizing Veeva the Platform for the Whole Suite of Regulatory Use Cases
There are numerous reasons why Veeva Vault has become a popular strategic platform for regulatory information management (RIM) and more–from its easy accessibility and scalability in the cloud, to its regular updates adding new innovative functionality.
However modest or ambitious Life Sciences companies’ plans to harmonize Regulatory content and standardize and streamline processes may be–potentially spanning adjacent functions such as Quality and Clinical operations–Veeva perfectly supports all scenarios with built-in futureproofing.
Common RIM Challenges
Life sciences companies often face several challenges in Regulatory Information Management (RIM) due to the complex nature of regulatory requirements, data management, and the need for compliance across different regions. A software solution such as Veeva Vault RIM can help address these challenges by providing streamlined processes, centralized data management, and compliance support. Here are a few of the common challenges that life sciences companies expect a RIM software solution to help address:
Complex Regulatory Requirements
Navigating the complex global regulatory landscape is challenging. Companies expect RIM software to provide updates on regulatory changes, guidelines, and standards to ensure compliance. The software should enable easier adaptation to regulatory requirements across various regions and countries.
Data Integration and Accessibility
Life sciences companies often deal with vast amounts of data from diverse sources, including clinical trials, research data, and regulatory submissions. A RIM solution should offer seamless data integration across various platforms and ensure that information is easily accessible, well-organized, and securely stored. This includes managing a wide range of document types and formats.
Efficient Submission Management
Preparing and managing regulatory submissions is a time-consuming process that involves compiling, organizing, and submitting large volumes of data. A RIM solution should streamline the submission process, provide templates and guidance for different types of submissions, and ensure that all documentation meets the specific regulatory standards of each region. The software should also allow tracking of submission status and deadlines to avoid compliance issues.
Collaboration and Communication
Collaboration among various departments and stakeholders is crucial in the regulatory process. RIM software should facilitate effective communication and collaboration by providing tools for project management, document sharing, and version control. This ensures all team members work with the most up-to-date information and can easily track changes and approvals.
Compliance and Risk Management
It is paramount to ensure compliance with regulatory requirements and minimize risks associated with regulatory actions. RIM software should offer robust compliance and risk management features, such as audit trails, compliance checks, and alerts for potential issues. This helps companies proactively address compliance risks and maintain a state of continuous regulatory readiness.
By addressing these challenges, RIM software can significantly improve the efficiency and effectiveness of regulatory processes, reduce the risk of non-compliance, and ultimately accelerate the time to market for life sciences products.
Veeva in a Regulatory Context
Veeva RIM (Regulatory Information Management) is a comprehensive suite designed to help life sciences companies manage their regulatory processes more efficiently. Here’s how Veeva RIM supports addressing the challenges mentioned:
Adapting to Complex Regulatory Requirements
Veeva RIM provides a unified platform for tracking regulatory requirements across different regions and countries, ensuring that companies can easily adapt to various regulatory landscapes. It includes regulatory intelligence features that offer insights and updates on the latest regulatory guidelines, helping companies stay compliant.
Streamlining Data Integration and Accessibility
The software offers a centralized repository for all regulatory documents and data, enabling easy access and management of information. It supports integration with other systems and platforms, ensuring that data is harmonized and efficiently retrieved, enhancing data accessibility and integrity.
Enhancing Efficient Submission Management
Veeva RIM simplifies the submission process through automation and structured workflows. It provides tools for creating, compiling, and managing regulatory submissions, including templates and checklists that align with specific regulatory requirements. The system also tracks submission statuses and critical deadlines, ensuring timely and compliant submissions.
Facilitating Collaboration and Communication
The platform enhances collaboration among internal teams and external partners. It offers document sharing, version control, and project management tools that enable effective teamwork and ensures all stakeholders are aligned and informed. This collaborative environment helps streamline the regulatory process and reduce errors.
Proactive Compliance and Risk Management
Veeva RIM includes risk management and compliance monitoring features, such as audit trails and automated compliance checks. These tools help identify potential compliance issues early and enable companies to take corrective actions promptly, reducing non-compliance risk and enhancing regulatory readiness.
By providing these capabilities, Veeva RIM addresses the critical challenges faced by life sciences companies in Regulatory Information Management, helping them to navigate the complex regulatory environment more effectively and efficiently.
fme & Veeva: Securing your RIM platform’s ROI
In conclusion, Veeva Vault’s RIM solutions address life sciences companies’ multifaceted challenges in regulatory information management. By providing a unified platform that adapts to complex regulatory requirements, streamlines data integration, enhances submission management, and facilitates collaboration, Veeva Vault empowers organizations to navigate the regulatory landscape efficiently.
fme is crucial in helping clients maximize their ROI in Veeva Vault. With expertise in data migration, deduplication, and enrichment, fme ensures that life sciences companies have a harmonized platform that serves as a single source of truth for regulatory information. fme’s comprehensive business consulting and strategic technical advice, coupled with its experience in system implementation and migration in regulated industries, enable clients to optimize their RIM environment’s performance. Furthermore, fme’s ability to lay out future-stage strategies for digital process transformation extends the benefits of Veeva Vault beyond the regulatory function to adjacent departments, driving the best possible outcomes and ensuring a significant return on investment.
To discuss or analyze a current RIM project or transformation requirement, contact us. We’d love to learn your challenges and discuss a specific requirement relating to Veeva Vault-based RIM.
 fme AG
fme AG fme SRL
fme SRL


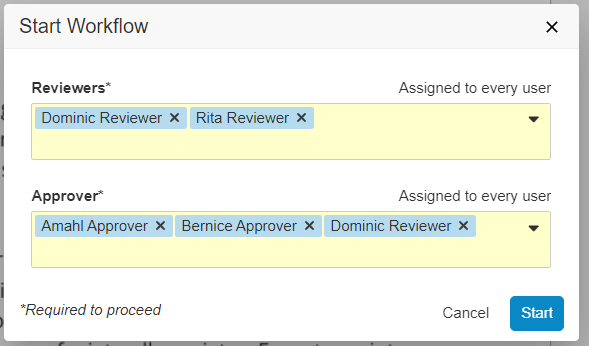
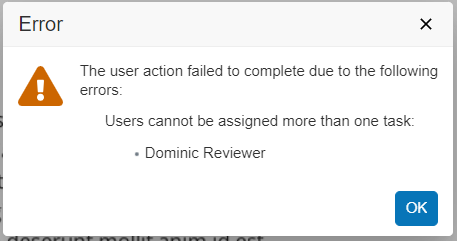
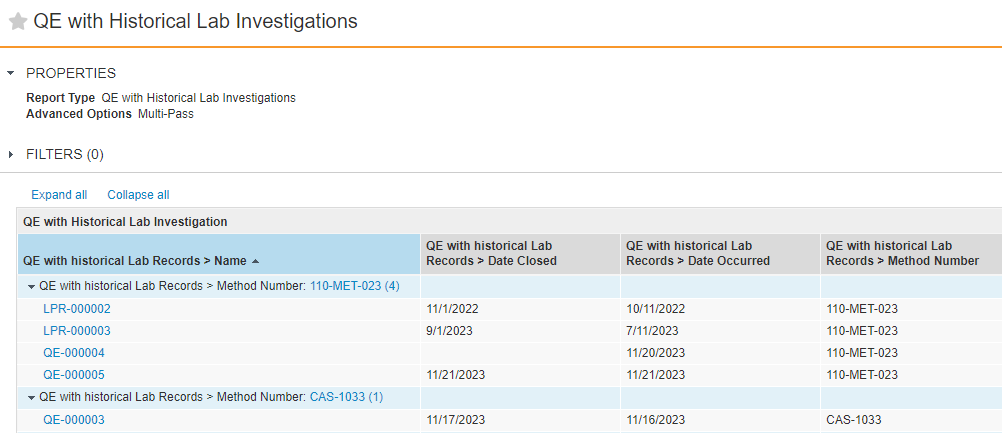

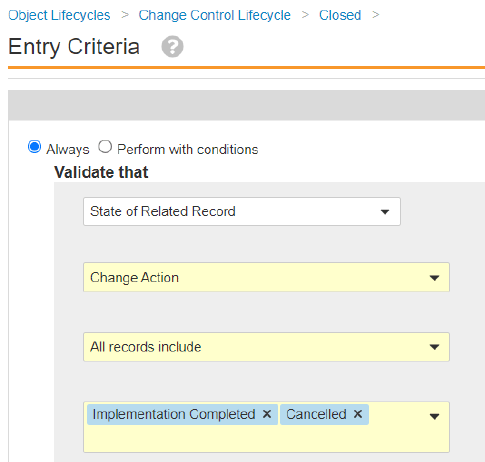
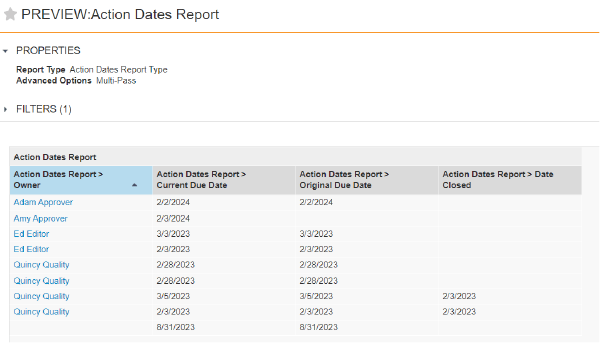
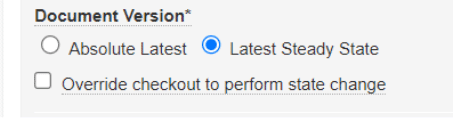 Scheduled Jobs can promote or demote documents based on configured criteria. In some situations, the scheduled job action on a document fails because the document is checked out. With this release, an Admin can configure the job to expire/obsolete a document that is in the Steady State despite having a minor version of the document checked out by ticking a checkbox within the job configuration page.
Scheduled Jobs can promote or demote documents based on configured criteria. In some situations, the scheduled job action on a document fails because the document is checked out. With this release, an Admin can configure the job to expire/obsolete a document that is in the Steady State despite having a minor version of the document checked out by ticking a checkbox within the job configuration page.