External Signatures with DocuSign in Veeva
Agile Integration with fme integration-center
About this Datasheet
Many business-critical processes in the life sciences and other regulated industries are highly reliant on timely document approvals, involving electronic signatures in a lot of cases. In today’s inter-connected business world, the approvers are not limited to signatures within the team or even within the company anymore, but increasingly these processes span across partner and supplier networks as well. Allowing these activities to complete effectively and efficiently can have a major positive impact on project.
A day in the life…
The internal team is collaborating on documents within the business application, such as Veeva. Once the documents are ready for approval they are sent to the internal approvers, who can easily approve and apply their electronic signature directly within the application – so far so good. But what about a scenario where the approvers are external to the company? Involving external approvers and signatories into internal processes can be challenging. Some commonly encountered scenarios include:
- Sponsor approvals for CRO Documents
- Customer approval of Specifications, Certificate of Analysis, Master Manufacturing Records for a contract manufacturer
- Partner signatures for project plans and reports
- Trainee signature for an in-person training event where attendees are not users within the LMS
Leveraging technologies like DocuSign is one option to support the use cases outlined above. However, connecting these technologies between your existing systems and maintaining compliance with Part 11 and Annex 11 can be challenging. fme’s integration-center framework allows robust and flexible connections between systems in your enterprise landscape with support for compliance and continuous improvement.
Built on future-proof technology
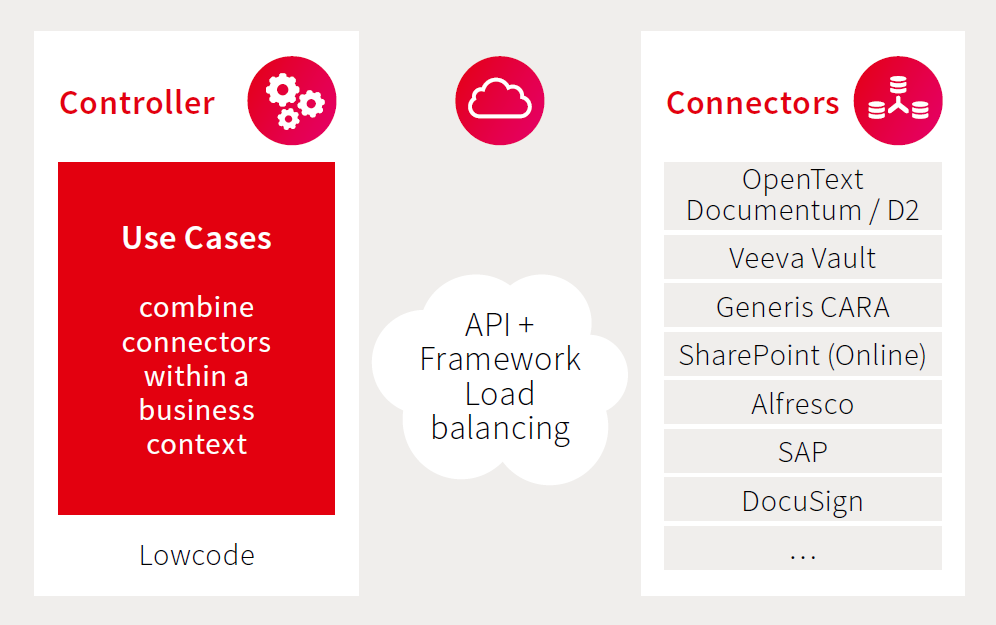
Take advantage of the benefits
- Accuracy – no disruption between the business application and the content shared externally
- Efficiency – no need for manual steps to share the documents for external approvals
- Traceability – Veeva workflow is showing the completion status within DocuSign
- Compliance – maintaining 21 CFR Part 11 requirements
- Security – documents are fully encrypted and protected within the DocuSign application
Contact Us
Don't like forms? Contact us directly to get more details on any of fme's services. We'd love to discuss your current challenges, and how fme can help you get the highest ROI and lowest TCO for your technology solutions. Call or use my personal contact form with your question, and I'll get you connected with the right fme expert.
fme is always looking for top talent to join our team. If you are looking to join the team you'll love to work with, review our job listings and review the details of current openings. Contact me if you have any questions on the application process.
 fme AG
fme AG fme SRL
fme SRL