Case Study
A manufacturing life sciences company is installing a new centrifuge for a production plant producing drug substances. In order to avoid operating errors, every employee who will operate the device in the future must undergo compulsory training tailored to the device. Within the training course, among other things, the work instructions created for the device are explained.
The work instruction passes through the obligatory document management processes prior to the training. Within the company’s own electronic document management system (DMS), those responsible for production operations approve the work instructions digitally. The approval takes place on 15 January. A document coordinator specifies February 1 as the effective date for the document. The device is to be used as of this date. The DMS ensures that the working instructions receive a corresponding electronic watermark automatically on February 1. Furthermore, the DMS automatically copies the watermarked document into the company’s own intranet (publishing).
Due to regulatory requirements, the work instruction may only be effective after all relevant employees have been trained by the DMS.
Two employees cannot attend the training due to illness. Since the training took place only a few days before the effective date and not all employees were able to complete the training, a responsible document coordinator must ensure that the document will not be effective from 1 February. Only when the remaining employees have completed the training, the work instructions may be effective. To do this, the coordinator sets a lock on the document: An Effectivity Hold. After the employees have been trained, the document coordinator removes the lock from the document. As soon as the lock is removed, the DMS transfers the work instruction to the effectivity status including the above-mentioned publishing.
Solution from the user’s point of view (excerpt)
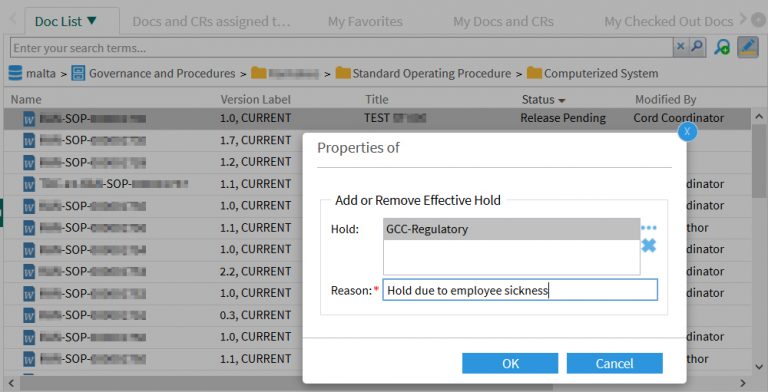
The document coordinator sets an Effectivity Hold to an approved work instruction.
A reason must be given that is recorded in the audit trail of the document:
Technical implementation (excerpt)
The outlined business process is implemented on the basis of the OpenTextDocumentum Life Sciences Solution Suite. Customer-specific adaptations at D2 level ensure that the Effectivity Hold functionality is seamlessly integrated into the Life Sciences Suite.
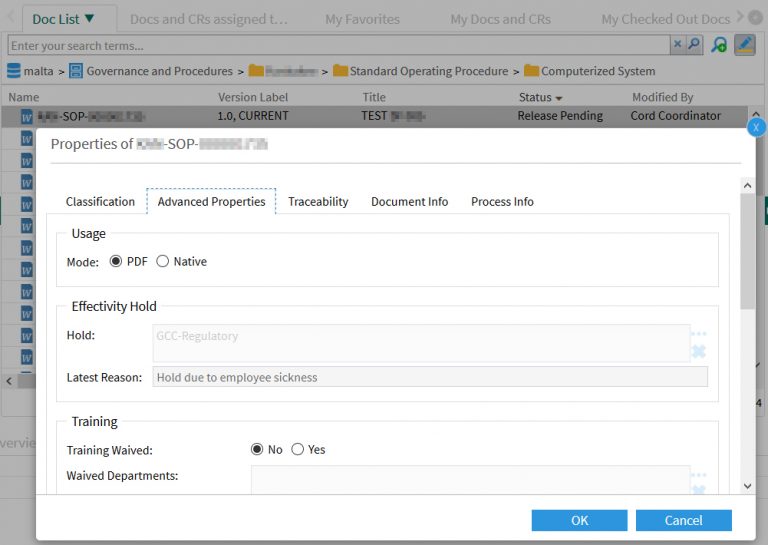
A D2 Property Page provides the input fields for the document coordinator:
Do you have any questions or wish further information?
Simply fill in our > contact form or visit our > OpenText Documentum Life Sciences Solution Suite landing page.
Here you can find the second part of my blogpost series »Special use cases based on OpenText Documentum for Life Sciences«: > The Mysterious Case of TDC
 fme AG
fme AG fme SRL
fme SRL
